
将不溶解和不熔融的多孔材料制备成凝胶,以应用于先进技术,具有重要意义但也面临巨大挑战。在这里,作者提出了一种通用、简便且可扩展的方法,利用基团保护合成策略制备共价有机框架(COF)凝胶。为验证该策略的普适性,我们成功制备了10种高结晶性、多孔性、机械性能良好、对溶剂和冻结具有优异抵抗能力的COF有机水凝胶。值得注意的是,这些COF有机水凝胶可以轻松转变为水凝胶、有机凝胶和气凝胶,消除了不同类型COF凝胶之间的差距。深入的机理研究揭示了基团保护策略有效减缓了COF的形成速率并调控了其形态,有利于形成交联的纳米纤维/纳米片,从而产生COF凝胶。作者还发现,在有机/水二元溶剂和COF骨架中的功能基团形成的氢键网络在创建有机水凝胶以及保持抗冻和溶剂抵抗性方面起着至关重要的作用。作为应用演示,携带光响应偶氮苯基团的COF凝胶显示出优异的太阳能吸收、光热转换和水传输性能,在太阳能海水淡化中具有巨大潜力。该工作丰富了COF凝胶的合成工具箱,并扩展了COF的应用范围。

Figure 1. (a) Illustration of the synthesis approach of COF gels. (b) COF gel monomers used in this study. (c) Structures and images for 10 different COF gels studied (TpPa, DHTA-Pa, TpBD, TpAzo, TAPB-Tp, NKCOF-58, NKCOF-59, NKCOF-1, NKCOF-60, NKCOF-61).
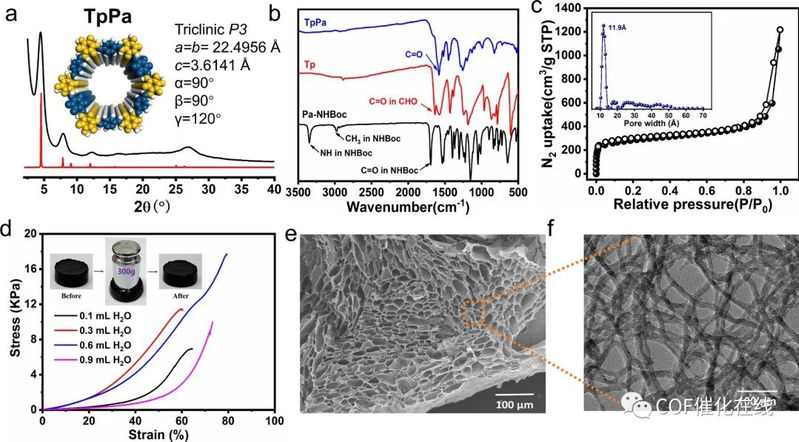
Figure 2. (a) Pawley refinements against the PXRD patterns of TpPa COF gel. (b) FT-IR spectra of TpPa COF gel compared with reactants. (c) Adsorption–desorption isotherms (inset: pore-size distribution) of TpPa COF gel. (d) Stress–strain curves of TpPa COF gel under different ratios of dioxane to water (inset: images of the COF gel compressed by 300 g weights). (e) SEM image of the hollow honeycomb superstructure with micron-sized pores in the freeze-dried TpPa COF gel. (f) TEM image of fiber-like structures of COF sheets.
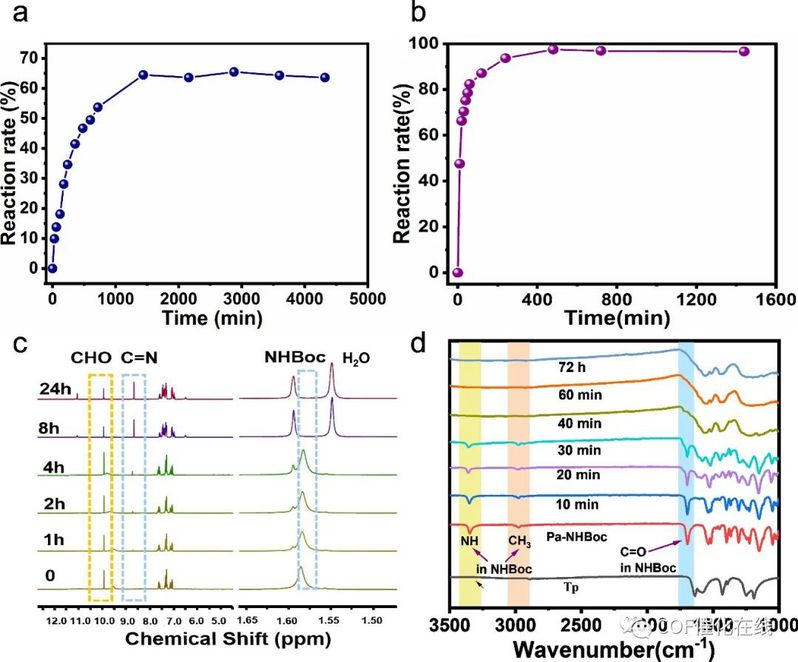
Figure 3. (a, b) Reaction rate of reactions a and b over time, respectively. (c) 1H NMR spectra of reaction a over time. (d) FT-IR spectra of experiment 3 over time.
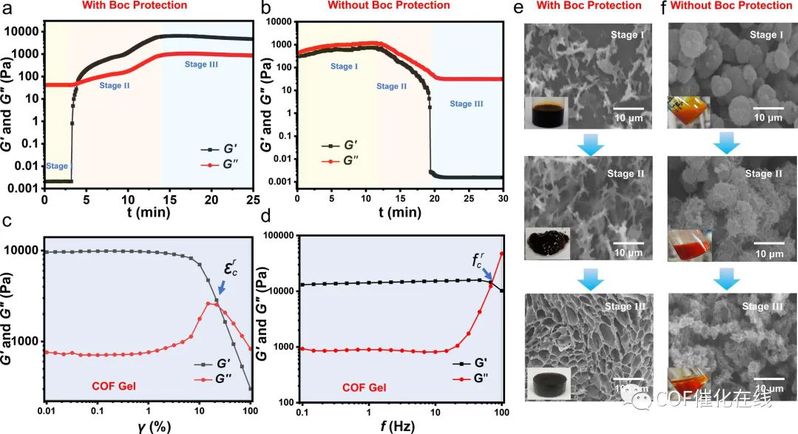
Figure 4. (a, b) Oscillatory time sweep profile of COF gel (a) and COF powder (b) formation in situ (under 120 °C). (c) Strain sweep γ measurements of the COF gels. (d) Frequency sweep f measurements of the COF gels. (e, f) SEM images of the COF gels (e) and COF powders (f) corresponding to each formation stage over time (inset: visual aspect).
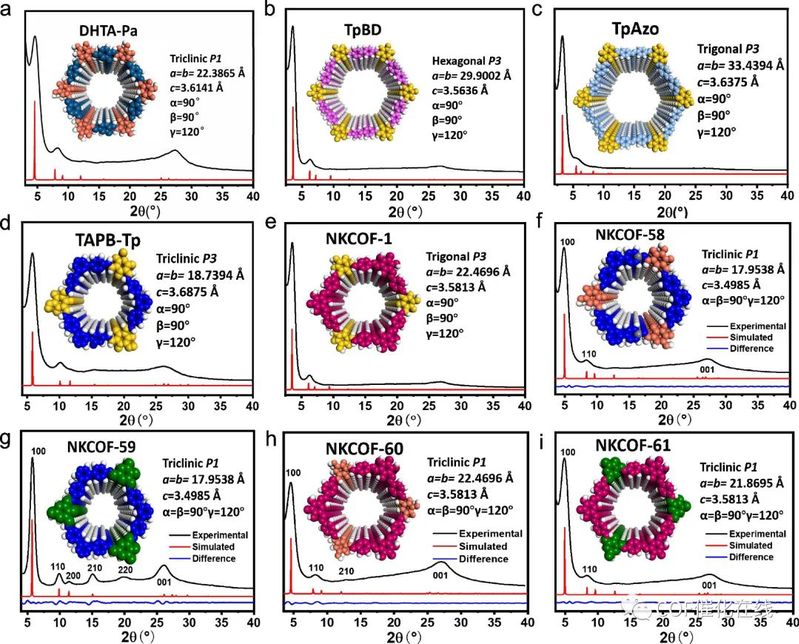
Figure 5. (a–e) The PXRD patterns of DHTA-Pa, TpBD, TpAzo, TAPB-Tp, and NKCOF-1 gels, respectively. Black lines: experimental PXRD data. Red lines: simulated PXRD of AA stacking. (f–i) Experimental PXRD pattern (black), simulated AA-stacking pattern (red), and the difference between the experimental and calculated data (blue) of NKCOF-58, -59, -60, and -61 COF gels.
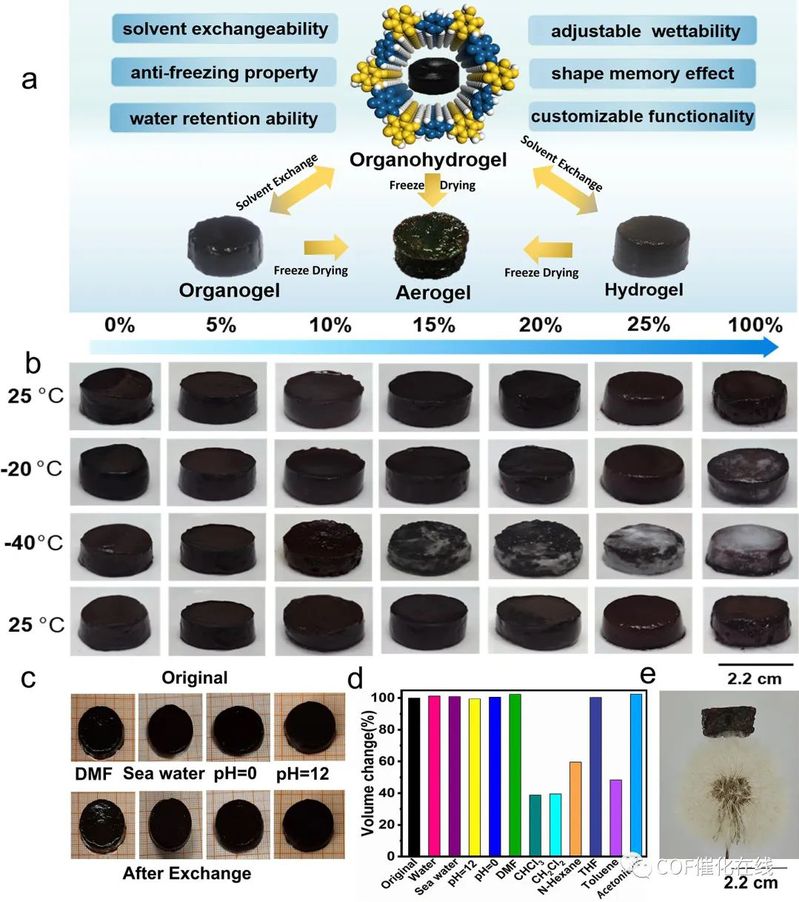
Figure 6. (a) The transformation between COF organohydrogels, COF hydrogels, COF organogels, and COF aerogels and advantages of COF organohydrogels. (b) The effect of water volume percentage (vol %) on the antifreezing performance of TpPa COF gels. (c) Swelling exhibitions of COF gels in different solvents after swelling for 3 days. (d) The swelling ratio of TpPa COF gels in various solvents. (e) Photographs of COF aerogels on the tomenta of a dandelion.
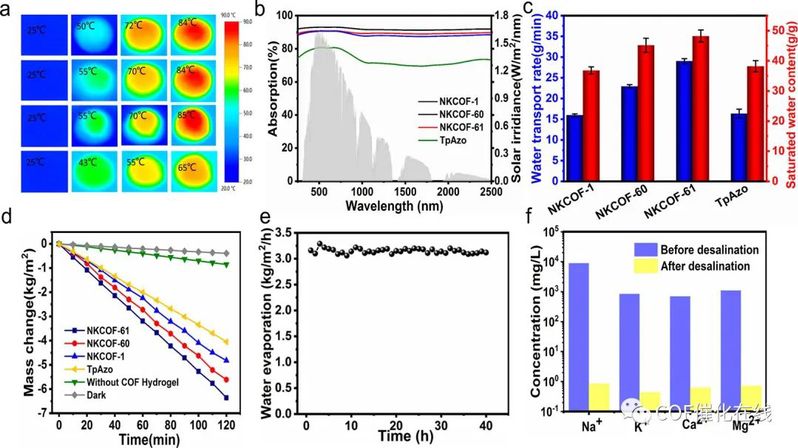
Figure 7. (a) Solar thermal images of different COF gels before and after 1 sun irradiation. (b) UV–vis–NIR absorption spectra of different COF gels. (c) Water transport rate and saturated water of different COF gels. (d) Mass change of pure water of different COF gels upon 1 sunlight irradiation (1 kW/m2). (e) Stability evaporation test applying pure water of NKCOF-61 gels over 40 h. (f) Ion concentration of water before and after desalination.
https://pubs.acs.org/doi/10.1021/jacs.3c09284
