
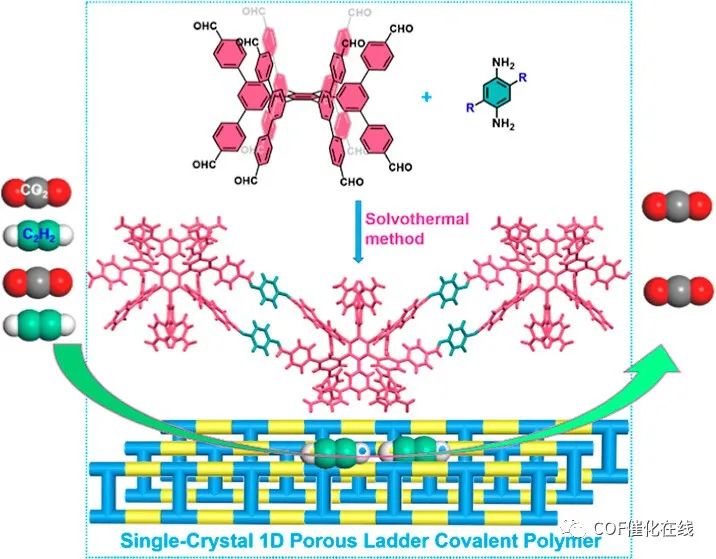
单晶、一维(1D)聚合物的合成非常重要,但也是一个巨大的挑战。在此,作者报道了通过动态共价化学在溶液中合成单晶1D梯形聚合物。三维电子衍射技术用于严格解析结晶聚合物的结构,揭示了每个聚合物链通过双共价桥连接,并且所有聚合物链通过π-π堆积和氢键相互作用以交错和交织的方式堆积,使得结晶聚合物在热和化学稳定性方面高度稳定。通过动态穿透实验验证,合成的单晶聚合物具有永久性微孔,能够有效去除C2H2/CO2混合物中的CO2,获得高纯度的C2H2。这项工作证明了在溶液中构建具有双共价桥的单晶1D多孔梯形聚合物用于有效分离C2H2/CO2的第一个例子。
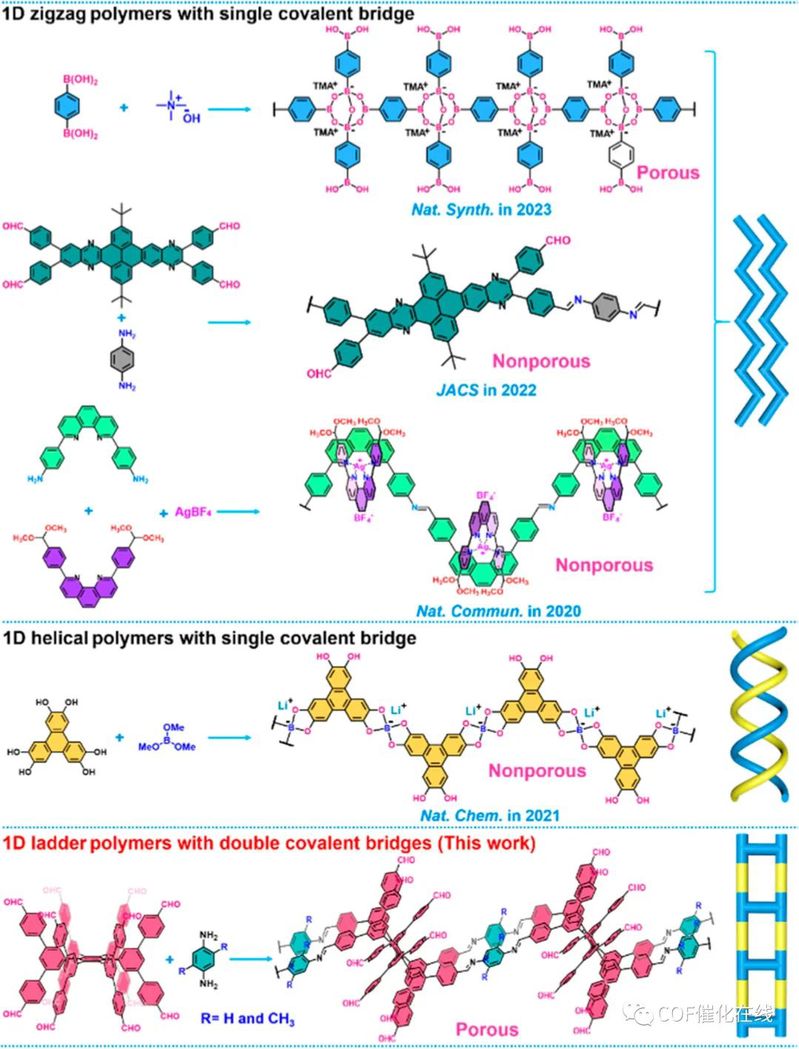
Scheme 1. Reported Examples of Synthesizing Single-Crystal 1D Covalent Polymers and Our Work
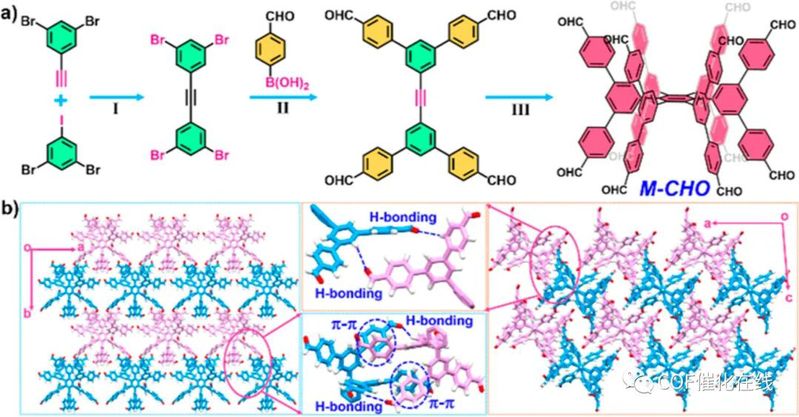
Scheme 2. (a) Synthetic Route of M-CHO and (b) Its Single-Crystal Structure: (I) Pd(PPh3)2Cl2, CuI, Et3N, THF, r.t., 24 h; (II) Pd(PPh3)4, K2CO3, THF, H2O, 85 °C, 12 h; (III) Co2 (CO)8, 1,4-Dioxane, 115 °C, 24 h
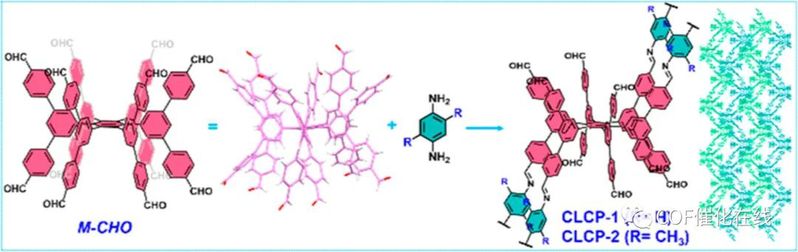
Figure 1. Synthetic route of CLCP-1 and -2 and their structure diagram.
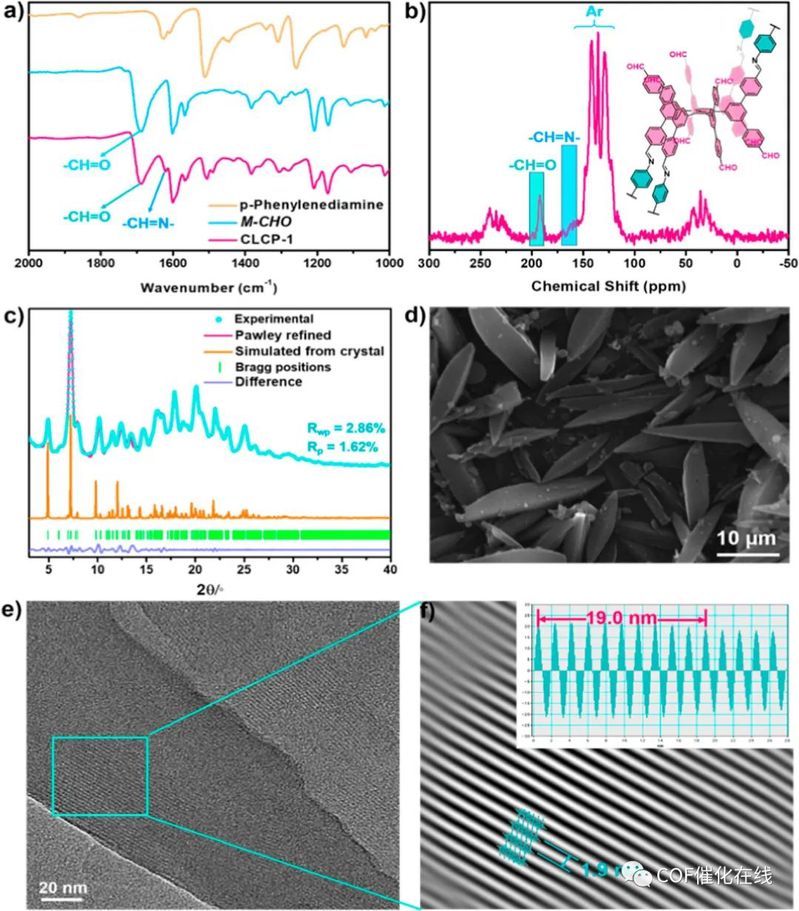
Figure 2. Characterization. (a) FT-IR spectra of CLCP-1. (b) 13C CP-MAS NMR spectra of CLCP-1. (c) PXRD patterns of CLCP-1. (d) SEM image of CLCP-1. (e) HR-TEM image of CLCP-1. (f) Partial enlarged view of (e).
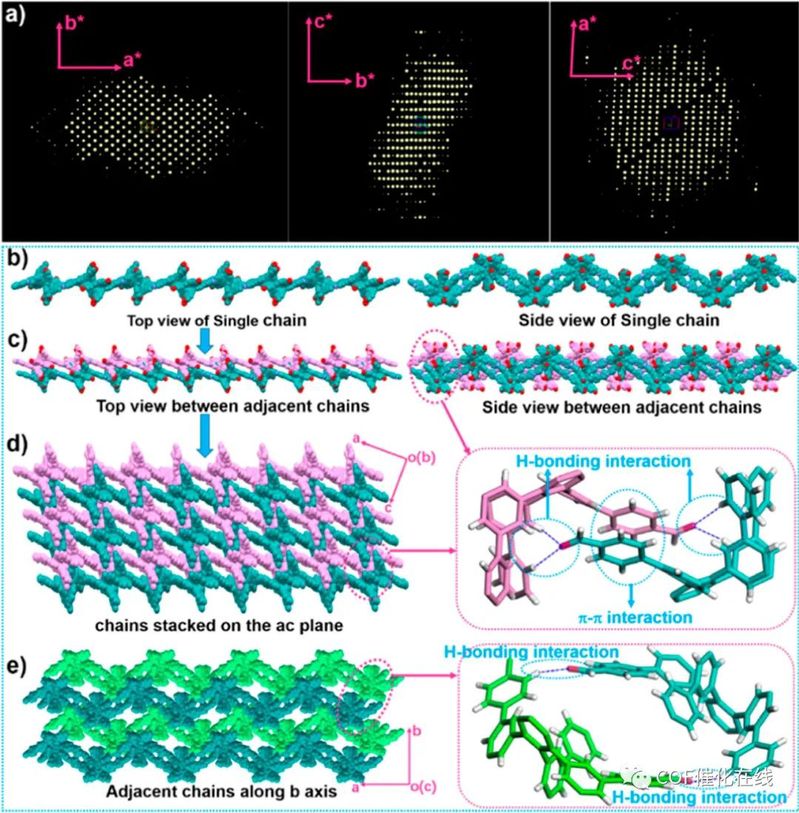
Figure 3. (a) 2D projections of the reconstructed reciprocal lattice of CLCP-1 from 3D ED data. (b) Single chain of CLCP-1. (c) Adjacent chains of CLCP-1 on the ac plane. (d) Chain stacking of CLCP-1 on the ac plane and the interaction between chains. (e) Adjacent chains of CLCP-1 along the b axis and the interaction between chains.
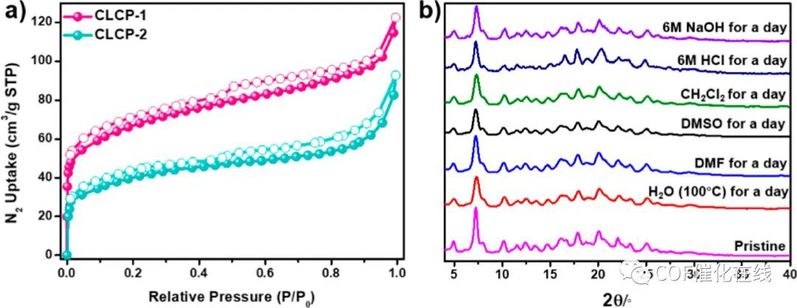
Figure 4. (a) N2 adsorption (solid symbols) and desorption (open symbols) isotherms at 77 K of CLCP-1 and -2. (b) PXRD patterns of CLCP-1 after treatment under various harsh conditions.
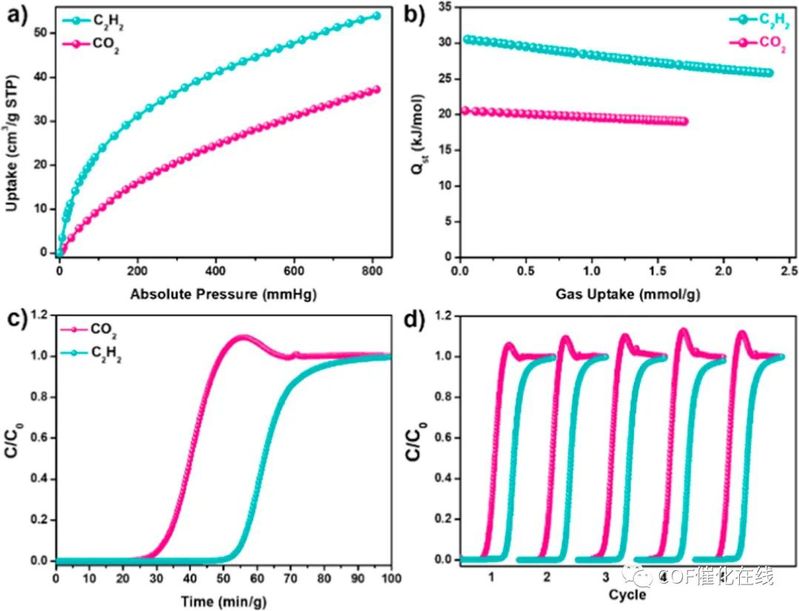
Figure 5. (a) Single-component adsorption isotherms of C2H2 and CO2 for CLCP-1 at 298 K. (b) Qst curves of C2H2 and CO2 for CLCP-1 at 298 K. (c) Experimental breakthrough curves of C2H2/CO2 mixtures (1/1, v/v) for CLCP-1 with a total inlet flow rate of 2.0 mL/min at 298 K. (d) Cyclic breakthrough curves of C2H2/CO2 mixtures (1/1, v/v) for CLCP-1 at 298 K under 1 bar.
https://pubs.acs.org/doi/10.1021/jacs.3c10812#
